Introduction
The pharmaceutical industry operates within a complex web of regulatory standards, with patient safety and public trust at the forefront. Compliance with regulations is crucial for pharmaceutical companies to ensure the safety of patients and maintain public trust. However, the industry faces numerous challenges, including strict regulations, frequent changes in laws, and severe penalties for non-compliance. This article explores the compliance and regulatory challenges faced by the pharmaceutical industry and offers strategies and digital solutions to navigate these challenges effectively.
In the pharmaceutical sector, adherence to drug and alcohol policies is of utmost importance for safeguarding public health. This article delves into the strategies necessary to ensure compliance with these policies in the pharmaceutical industry. It highlights the importance of clear policies, employee training, monitoring systems, temperature mapping, digital solutions, and a resilient compliance team. By implementing these strategies, pharmaceutical companies can protect patient health, maintain compliance, and avoid costly legal complications.
Digital solutions play a vital role in managing compliance issues in the pharmaceutical industry. This article discusses how these tools automate and streamline compliance processes, mitigate the risk of human errors, and provide real-time monitoring and alerts. It also emphasizes the significance of effective documentation management and tracking regulatory changes. By leveraging digital solutions, pharmaceutical companies can enhance their compliance management systems and navigate the complex landscape of regulatory compliance.
The successful implementation of compliance measures in the pharmaceutical sector is explored through case studies. These case studies highlight the role of digital solutions in achieving regulatory compliance and improving operational efficiency. By partnering with software development companies and adopting tailored compliance management systems, pharmaceutical companies can streamline processes, reduce compliance risks, and build trust with stakeholders.
Lastly, the article examines future trends in compliance regulations in the pharmaceutical industry. It emphasizes the need for organizations to stay ahead of regulatory changes by investing in adaptable digital solutions and cultivating a compliance culture. The importance of a robust regulatory change management framework and AI-powered platforms is discussed, along with case studies showcasing their effectiveness. By embracing these strategies and technologies, pharmaceutical companies can navigate regulatory changes efficiently and ensure the safety and quality of their products.
1. Understanding Compliance and Regulatory Challenges in the Pharmaceutical Industry
The pharmaceutical sector is a labyrinth of regulatory standards, with patient safety and public trust at the fulcrum. Operating within this intricate web, pharmaceutical companies must ensure not just adherence to regulations, but also the safety of patients and the maintenance of public trust. The industry is fraught with challenges such as strict regulations, frequent changes in laws, and severe penalties for non-compliance. These challenges are only heightened by the global nature of the industry, where regulatory standards differ across jurisdictions.
A white paper by MasterControl Inc., a quality and compliance management solutions provider, identifies six significant oversights that can be propagated over time in pharmaceutical companies. These oversights include inadequate investments in Contract Research Organizations (CROs), insufficient training of corporate personnel on quality and compliance standards, and failure to invest in technologies that enhance quality and compliance processes. A lack of understanding or investment in pharmacovigilance and pharmacoepidemiology, crucial for monitoring the safety and effectiveness of pharmaceutical products, is another oversight. Risk-based management, which involves identifying and mitigating potential risks to quality and compliance, is often overlooked. The absence of necessary quality and compliance personnel to ensure regulations' adherence is a significant oversight.
ISPE (International Society for Pharmaceutical Engineering) offers valuable resources for pharmaceutical professionals to tackle these issues. They produce guidance documents offering practical information on industry best practices and regulatory expectations. Guides from the GAMP (Good Automated Manufacturing Practice) series focus on achieving compliant computerized systems and cover a wide range of topics, including data integrity and manufacturing records. ISPE also offers good practice guides (GPGs) that cover various topics such as particulate containment, asset management, booklet labels, clinical supply systems, cold chain management, comparator management, and more. Baseline guides establish a compliant minimum acceptable baseline approach to specific topic areas, such as active pharmaceutical ingredients, oral solid dosage forms, sterile product manufacturing facilities, water steam systems, commissioning qualification, biopharmaceutical manufacturing facilities, risk-based manufacture of pharma products, and Pharma 4.0. The APQ (Advancing Pharmaceutical Quality) guide series focuses on assessing and improving an organization's quality management maturity, covering topics such as change management, cultural excellence, management responsibilities review, and process performance product quality monitoring system.
Pharmaceutical companies often implement various strategies to ensure compliance.
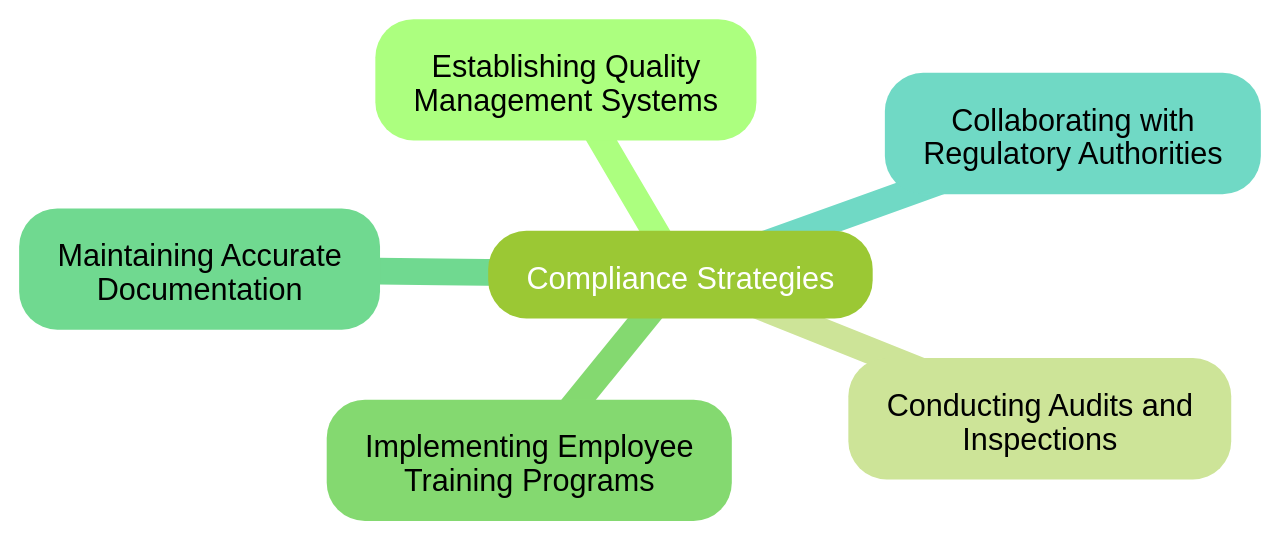
These include establishing robust quality management systems, conducting regular audits and inspections, implementing comprehensive employee training programs, and maintaining accurate and up-to-date documentation of processes and procedures. Companies also collaborate and partner with regulatory authorities to stay updated on the latest industry regulations and guidelines.
Patient safety regulations are crucial to ensure the safety and well-being of patients who use pharmaceutical products. These regulations govern various aspects of the industry, such as drug development, manufacturing, labeling, and distribution. Compliance with these regulations is a legal requirement, and failure to adhere can result in severe consequences, including fines, product recalls, and damage to the company's reputation.
Non-compliance penalties in the pharmaceutical industry can significantly impact a company. These penalties can include fines, sanctions, and legal action. The exact impact can vary depending on the violation's severity and the company's size, but in general, non-compliance penalties can damage a company's reputation, lead to financial losses, and even result in the suspension or revocation of licenses.
Regulatory challenges in the pharmaceutical industry can be complex and varied.
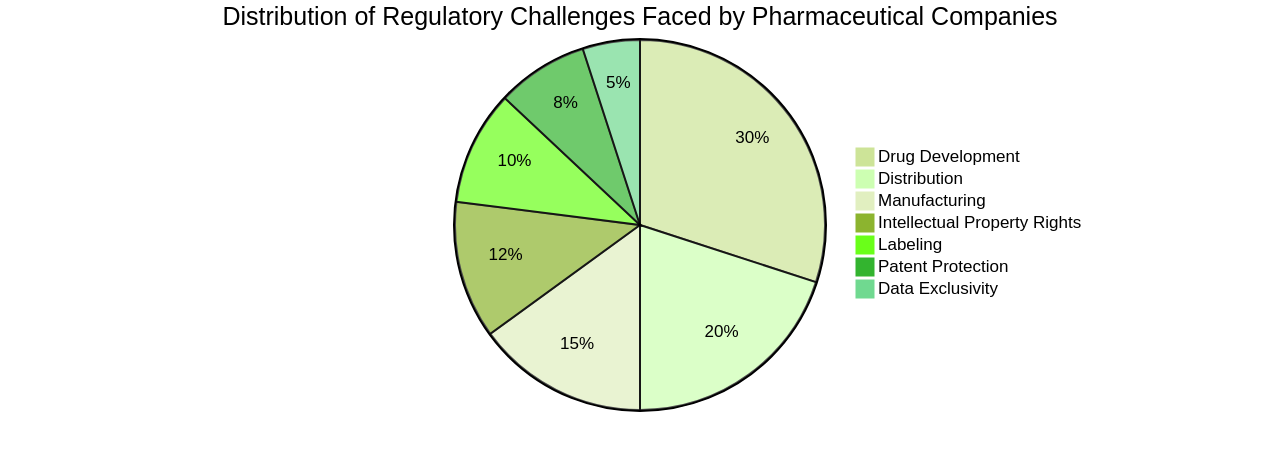
Companies in this industry often face stringent regulations and guidelines imposed by government agencies to ensure the safety and efficacy of their products. Compliance with these regulations is crucial to avoid legal and financial consequences. Pharmaceutical companies must navigate regulatory processes for drug development, clinical trials, manufacturing, distribution, and marketing.
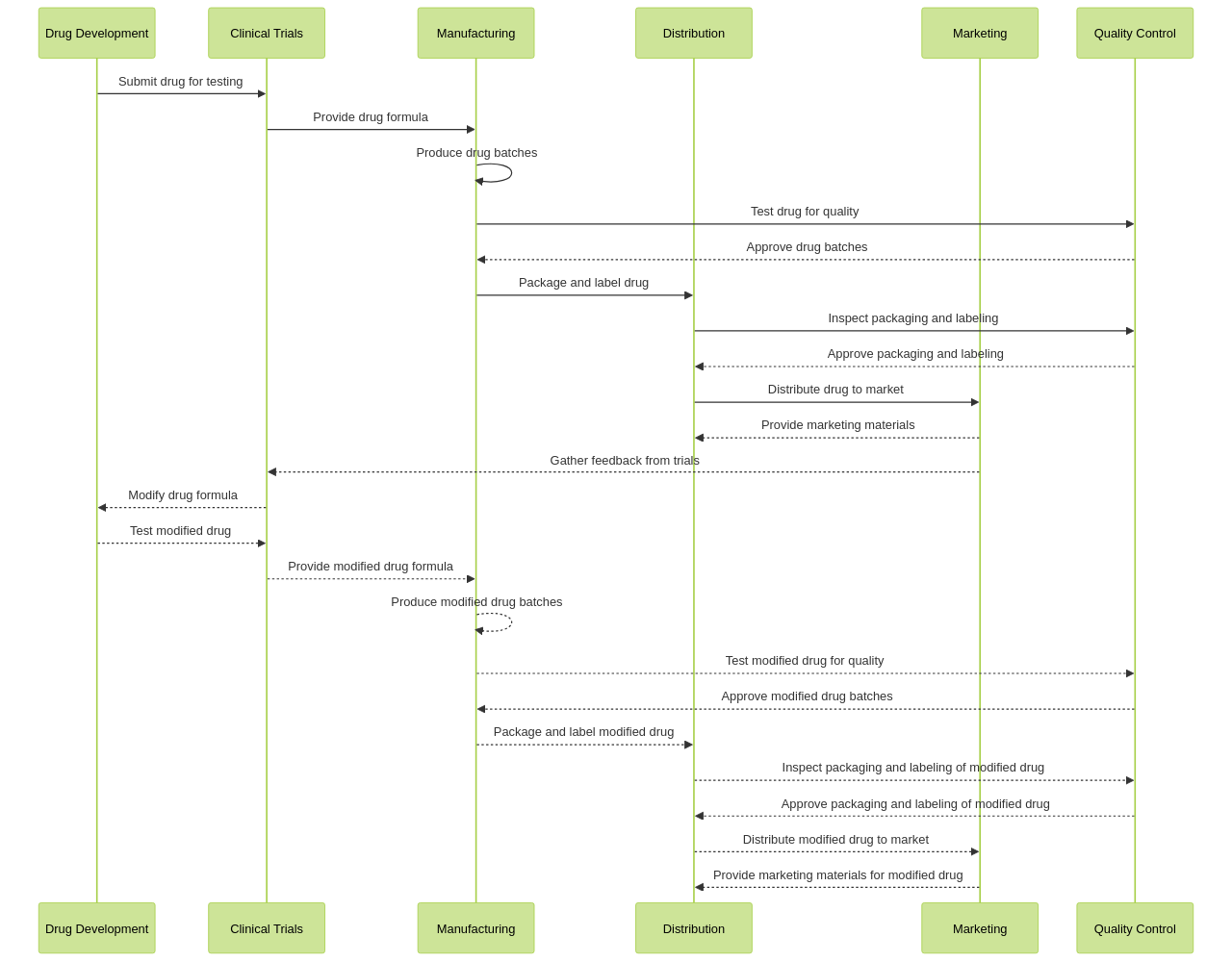
They need to demonstrate the safety and effectiveness of their products through rigorous testing and documentation. Additionally, they must adhere to strict quality control standards and meet labeling and packaging requirements. Regulatory challenges also extend to intellectual property rights, patent protection, and data exclusivity. To overcome these challenges, pharmaceutical companies often employ regulatory affairs professionals who specialize in interpreting and implementing regulations. They work closely with regulatory agencies to ensure compliance and navigate the approval processes. Continuous monitoring of regulatory changes and staying updated with industry guidelines is crucial for pharmaceutical companies to maintain compliance.
In the pharmaceutical industry, ensuring trust and patient safety is paramount. Companies must implement rigorous quality control measures and adhere to regulatory standards. This includes conducting thorough testing and analyses of pharmaceutical products to ensure their efficacy, safety, and quality. Additionally, maintaining transparent communication with healthcare professionals and patients, providing accurate and up-to-date information about medications, and promptly addressing any safety concerns or adverse events are essential steps in ensuring trust and patient safety. Regular audits and inspections by regulatory authorities play a significant role in verifying compliance with industry regulations and standards.
In conclusion, navigating the complex regulatory environment in the pharmaceutical industry requires expertise and knowledge of the relevant laws and regulations. By consulting with experts and staying updated on the latest regulations, companies can ensure that their products and services meet the necessary standards and comply with all applicable regulations. Partnering with experienced professionals who specialize in regulatory compliance can provide valuable guidance and support throughout the development process.
2. Strategies for Navigating Drug & Alcohol Policy Compliance
In the realm of the pharmaceutical industry, maintaining stringent adherence to drug and alcohol policies is of utmost importance. This is not merely a legal obligation but a crucial step towards safeguarding public health. A well-rounded approach to regulatory compliance necessitates the formulation of explicit policies, consistent employee training, and robust monitoring and auditing systems. Moreover, staying updated with regulatory shifts and tweaking policies in line with them is essential.
A case in point that highlights the dire consequences of non-compliance is the opioid crisis in the United States, largely instigated by Purdue Pharma's misrepresentation of OxyContin as non-addictive. This led to criminal and civil penalties totaling billions of dollars for Purdue Pharma and its top executives, not to mention the immense suffering inflicted on communities nationwide and the eventual bankruptcy of Purdue Pharma.
To circumvent such disastrous outcomes, pharmaceutical companies must prioritize investments in a robust compliance infrastructure. One key element of this is cold chain management, which entails storing and transferring medical products under specific conditions to preserve their effectiveness. A vital instrument in assuring cold chain compliance is the data logger, a compact electronic device that collates environmental data such as temperature, humidity, and differential pressure.
Data loggers bring several advantages to the table, including automated data collection, accuracy, and internal storage capability. Some regulators even require their use and stipulate the models or specifications that must be employed. Internet-enabled data loggers can send data via Bluetooth or Wi-Fi connections, allowing managers to monitor data in real-time and receive automatic alerts in response to specific environmental conditions.
Apart from monitoring, pharmaceutical companies also need to guarantee the consistency of storage conditions throughout their facilities. This is achieved through temperature mapping, which involves placing data loggers at various points in a facility to generate a temperature map. This assists in identifying temperature variations within the facility, ensuring that all products are stored within safe temperature ranges.
Digital solutions can significantly boost the efficiency and effectiveness of these processes. For instance, cloud storage offers cost and scalability benefits for storing data gathered by data loggers. However, companies must also be mindful of the cybersecurity risks associated with cloud storage.
Lastly, a solid compliance team is the backbone of any robust compliance strategy. This team should comprise healthcare professionals and should contemplate obtaining medical affairs board certification. By harnessing technological tools and building a formidable compliance team, pharmaceutical companies can ensure unwavering compliance with drug and alcohol policies, thereby safeguarding patient health and steering clear of expensive legal complications.
In essence, pharmaceutical companies must adopt a proactive stance towards compliance, utilizing a plethora of strategies and tools that encompass clear policies, employee training, monitoring systems, temperature mapping, digital solutions, and a resilient compliance team. In doing so, they can guarantee the safety and efficacy of their products, protect patient health, and evade the severe consequences of non-compliance.
3. Role of Digital Solutions in Managing Compliance Issues
The pharmaceutical industry, with its intricate web of regulatory compliance, leans heavily on digital solutions. These tools form the bedrock for managing complex compliance challenges, ensuring companies maneuver through regulatory intricacies with ease. Digital solutions offer a holistic approach, enabling firms to automate and streamline their compliance processes, thus mitigating the risk of human errors.
Let's take environmental monitoring in pharmaceutical operations as an example. This aspect is crucial for ensuring the quality and safety of products. It involves data collection on parameters such as temperature, humidity, and pressure to prevent degradation or contamination of sensitive products. Digital data loggers have emerged as an effective tool in this regard, automating the process of environmental monitoring, data archiving, tracking sensor calibration, and other critical functions. These systems can even send alerts in case of deviations, ensuring immediate corrective action.
Moreover, cloud-based monitoring systems have added a new dimension to compliance management. They allow remote access to data, offering a higher level of convenience and efficiency. Companies can monitor their compliance status in real-time, enabling them to respond swiftly to any potential issues.
Another critical aspect of digital solutions in the realm of compliance is their ability to manage documentation effectively. Compliance in the pharmaceutical industry requires stringent record-keeping and clear audit trails. Digital solutions can simplify this process, ensuring that all necessary documentation is easily accessible and well-organized.
Furthermore, these systems can assist in tracking regulatory changes, a particularly challenging task given the ever-evolving nature of regulations. This is particularly relevant in the context of the pharmaceutical industry, where regulations such as 21 CFR 11, 203, 205, and 211, enforced by the FDA, govern compliance.
Advanced analytics is another feature that digital solutions offer, which can be instrumental in compliance management. These analytics can provide insights into compliance risks, enabling companies to make informed decisions and implement proactive measures.
Employee training is another area where digital solutions can make a significant impact. They can support training programs, ensuring employees are aware of compliance requirements and are equipped to adhere to them.
In essence, digital solutions can play a pivotal role in managing compliance issues. From automating processes and managing documentation to supporting training programs and providing advanced analytics, these tools can significantly enhance a company's ability to navigate the complex landscape of regulatory compliance in the pharmaceutical industry.
4. Case Study: Successful Implementation of Compliance Measures in the Pharmaceutical Sector
The pharmaceutical industry is a competitive arena where digital solutions are increasingly playing a critical role in managing compliance and enhancing operational efficiency. The experiences of Aspire Pharma Limited (APL), a UK-based mid-sized pharmaceutical company, and Bigfinite, a San Francisco-based startup, serve as prime examples of this trend.
APL, primarily engaged in conducting small bioequivalence clinical trials, was seeking a robust long-term data management solution to ensure compliance with stringent industry regulations. This led to a partnership with Arkivum, a provider of digital preservation solutions for data integrity in life sciences. Arkivum's solution, which was tailored to APL's specific needs, provided both manual and automatic digital preservation options. This collaboration resulted in APL achieving Good Clinical Practice (GCP) and regulatory compliance in long-term data management. Consequently, APL successfully managed compliance documentation, tracked regulatory changes, and automated these processes. The solution also offered real-time alerts on regulatory changes, reducing compliance risks and improving operational efficiency.
Similarly, Bigfinite's software platform, Bigengine, has been instrumental in transforming biotech and pharmaceutical industrial processes. Bigengine leverages Amazon Web Services (AWS) to analyze large volumes of data in a secure and advanced environment. The software employs advanced analytical techniques like machine learning, artificial intelligence, and neural networks for real-time predictions and modeling. As a result, it has been successful in process optimization, cost reduction, anomaly detection, and product quality enhancement in pharmaceutical manufacturing.
The experiences of both APL and Bigfinite demonstrate the critical role of digital solutions in advancing compliance measures in the pharmaceutical industry. Companies can automate processes, receive real-time alerts on regulatory changes, and support risk assessment and auditing processes with these solutions. Furthermore, these digital solutions are proving to be revolutionary in reducing compliance risks and improving operational efficiency.
In the pursuit of similar success, pharmaceutical companies can consider partnering with software development companies that specialize in developing tailored solutions for compliance management systems. These firms offer consultation services to address any questions or concerns and can assist with developing and implementing a compliance management system that meets regulatory requirements and industry standards. Moreover, adopting software solutions for automated compliance documentation management can provide features like document storage, version control, automated reminders, and collaboration tools to streamline the compliance documentation process.
In summary, the implementation of digital compliance management systems in the pharmaceutical industry offers several benefits. It ensures the industry's adherence to all relevant regulations and guidelines, crucial for maintaining the safety and efficacy of pharmaceutical products. It also streamlines processes, improves operational efficiency, mitigates the risk of non-compliance, and potential legal issues. By implementing such systems, pharmaceutical companies can demonstrate their commitment to regulatory compliance and build trust with stakeholders, including customers and regulatory authorities.
5. Future Trends: Evolving Compliance Regulations and How to Stay Ahead
The pharmaceutical industry's landscape is ever-evolving, propelled by constant regulatory changes. Keeping pace with these developments is a must for organizations aiming to retain their competitive edge. It's not just about tracking regulatory changes but also about investing in adaptable digital solutions that can flex with the industry's shifts. Cultivating a compliance culture is equally vital, where all employees understand the importance of compliance and their role in upholding it.
A robust regulatory change management framework is an essential tool in this endeavor. It allows companies to navigate the challenges associated with regulatory changes. For instance, some professionals in compliance roles have reported taking up to a year to implement regulatory changes, pointing to the need for more efficient systems. This is where digital solutions developed by experts like those at BestToolbars.net come into play. These solutions can help in tracking and analyzing regulatory updates from diverse sources in real-time, enabling companies to respond swiftly to changes.
Standardization of regulatory taxonomy can also aid in streamlining the process by enhancing communication and categorization of updates. Clear definitions of roles and responsibilities of compliance professionals ensure accountability and facilitate the implementation process. Each regulatory update should be assessed in terms of its business impact. Detailed impact analyses identify affected risks, controls, policies, procedures, training, and reports, ensuring a comprehensive understanding of the change.
AI-powered platforms like Reg Review, developed by HekaAI, are also effective tools for streamlining compliance processes. They offer real-time screening of regulatory sources and AI-powered analysis of publications, as well as end-to-end management of compliance tasks. Automating the regulatory watch and compliance processes allows compliance leaders to integrate compliance into business processes and improve efficiencies.
Case studies highlight the effectiveness of such platforms. A multinational banking and financial services company based in Singapore implemented Reg Review and saw substantial time savings and improved efficiency in handling regulatory updates. Similarly, a leading Japanese bank implemented Reg Review across six Asian countries in three languages, resulting in a 76% reduction in processing time while maintaining a comprehensive audit trail for regulators.
In this rapidly changing regulatory environment, maintaining a proactive approach to managing regulatory changes, leveraging technology, and fostering a culture of compliance are critical strategies. These practices enable pharmaceutical companies to stay ahead and ensure the safety, efficacy, and quality of their products in an ever-evolving regulatory landscape.
Conclusion
The pharmaceutical industry operates within a complex web of regulatory standards, with patient safety and public trust at the forefront. Compliance with regulations is crucial for pharmaceutical companies to ensure the safety of patients and maintain public trust. However, the industry faces numerous challenges, including strict regulations, frequent changes in laws, and severe penalties for non-compliance. The implementation of robust compliance measures, such as clear policies, employee training programs, monitoring systems, temperature mapping, digital solutions, and a resilient compliance team, is essential to navigate these challenges effectively.
Digital solutions play a vital role in managing compliance issues in the pharmaceutical industry. These tools automate and streamline compliance processes, mitigate the risk of human errors, and provide real-time monitoring and alerts. Effective documentation management and tracking regulatory changes are also significant aspects facilitated by digital solutions. By leveraging these technologies, pharmaceutical companies can enhance their compliance management systems and navigate the complex landscape of regulatory compliance successfully.
In conclusion, navigating compliance and regulatory challenges is paramount in the pharmaceutical industry to ensure patient safety and maintain public trust. By implementing robust compliance measures and leveraging digital solutions, companies can protect patient health, reduce compliance risks, improve operational efficiency, and build trust with stakeholders. It is crucial for organizations to stay ahead of regulatory changes by investing in adaptable digital solutions and cultivating a strong compliance culture. Embracing these strategies will enable pharmaceutical companies to navigate regulatory changes efficiently while ensuring the safety and quality of their products.





